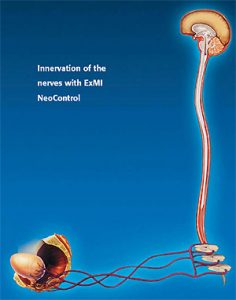
Extracorporeal Magnetic Innervation (ExMI™)
is based on the principle described by Faraday’s Law of Magnetic Induction. In the mid-1800s, Faraday demonstrated that muscular contractions could be induced by the application of a time-varying magnetic field.
ExMI technology is a highly advanced and sophisticated implementation of this basic principle, designed to help physicians manage the complex medical problem of urinary incontinence.
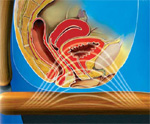
ExMI technology works by producing a highly focused, time-varying magnetic field that penetrates deep into the perineum, innervating the pelvic floor muscles by activating motor neurons. Pulses of steep gradient magnetic flux are produced by the therapy head.
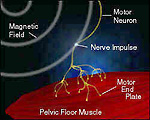
These fields penetrate the patient’s perineum and initiate nerve impulses. The time-varying magnetic field creates an electrical potential, which causes an ion flow, or Eddy currents, in the tissues. This ion flow results in a depolarization of resting motor neuron membrane potentials. When a threshold potential is reached, an action potential is initiated for that neuron. This action potential then propagates naturally along the axon via the usual Na+ and K+ ion flows. Once these impulses reach the motor end plates, the musculature of the pelvic floor responds by contracting at a rate equal to the output pulse rate of the therapy head. The muscles contract and relax with each pulse. If the output pulse rate exceeds that of the muscles‘ ability to contract and relax, the result is a constant, or steady, contraction of the muscles.
Simply put, ExMI technology in duces nerve impulses, which cause muscle contractions and increase circulation.
What else can ExMI do?
In recent years, dozens of clinical studies in 15 countries have been implemented regarding future applications of this technology. The basics of this technology listed above, along with the fact that such muscle activity radically improves blood flow, indicate a variety of additional targets for this therapy. Many of the following conditions are pending FDA approval* for treatment with ExMI: feces incontinence, prostatitis/prostadynia, interstitial cystitis, radical prostatectomy, benign prostatic hypertrophy (BPH), post-partum incontinence, female sexual dysfunction, male hormone and sperm count deficiency, pelvic pain in men and women, neuropathy due to chemotherapy/diabetes, and arthritis.